The Bertarelli Foundation has awarded collaborative research grants to four teams of scientists representing Harvard Medical School, its affiliated teaching hospitals, and the Institute of Molecular and Clinical Ophthalmology in Basel, Switzerland, all focused on understanding and treating some of the most devastating sensory disorders, including deafness, blindness and pain.
The grants are designed to foster cross-disciplinary cooperation among leading basic, translational and clinical neuroscientists in an effort to propel discoveries from laboratory to clinic.
“Neuroscience is experiencing an exciting confluence of two advances: an explosion in our understanding of how the brain works and how it goes wrong in neurological disease and the staggering arsenal of new biological tools that can modify genes and cells to treat disease,” said David Corey, the Bertarelli Professor of Translational Medical Science at HMS.
“The new projects of the Bertarelli Program will combine these advances to develop new therapies for debilitating sensory disorders,” Corey said.
The three-year grants, which provide $300,000 in funding per project per year, are part of the Bertarelli Program in Translational Neuroscience and Neuroengineering.
Established in 2010, based at HMS and led by Corey, the program brings together scientists from a range of disciplines to help bridge the gap between basic and translational neuroscience and to address important research challenges that, once solved, promise to have life-altering outcomes for patients with sensory disorders.
The program was conceived by Ernesto Bertarelli as “a fusion of different talents, passions and visions united by a commitment to find groundbreaking ways to treat people and to make their lives better.”
“Sensory disorders represent a vital frontier in neuroscience, both because of the extent to which they affect people’s lives all over the world but also because treatments for many of them feel within our grasp,” Bertarelli said. “These four new collaborative research projects are cases in point. I am excited to welcome them to the Bertarelli Program and to follow their progress as, together, we work towards the ultimate goal: clinical solutions that will change people’s lives.”
The latest round of funding from the Bertarelli Foundation brings to 15 the total number of research grants awarded since 2010.
The four newly awarded grants are:
Toward a therapy for deafness and blindness in Usher syndrome
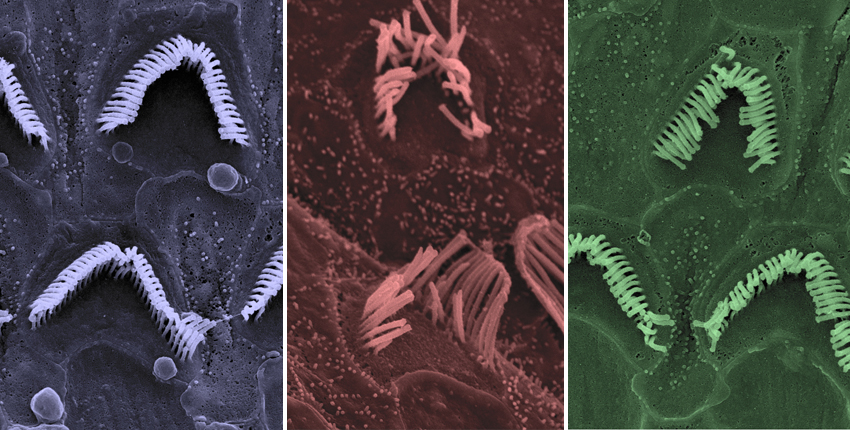
Two HMS neurobiologists studying the origins of deafness—Corey and Artur Indzhykulian, HMS assistant professor of otolaryngology at Massachusetts Eye and Ear—are joining forces with Botond Roska, an expert on retinal biology and eye disease at the Institute of Molecular and Clinical Ophthalmology in Basel, Switzerland, to develop treatments for a form of Usher syndrome. This genetic disorder arises from mutations in multi-tasking genes involved in the senses of hearing, balance and vision. Usher syndrome occurs in about 1 of 10,000 people and accounts for half of all inherited cases of combined deafness and blindness, according to National Institute on Deafness and Other Communication Disorders.
Corey and Indzhykulian’s work will focus on a particularly severe form of the disease, known as Usher syndrome type IF, characterized by profound deafness and absence of balance function at birth, along with progressive blindness beginning in a person’s 20s.
Because children with this form of the disease rely on their vision to compensate for their deafness and lack of balance, the eventual loss of sight can be particularly devastating.
The researchers will focus on developing gene therapy aimed at overcoming a hurdle that has stymied therapeutic efforts so far: the unusually large Usher 1F protein. Typically, researchers use a harmless virus, such as the common adeno-associated virus, as a delivery vehicle to carry a healthy copy of a gene into the target cells.
In this case, the targets are hair cells in the inner ear and photoreceptor cells in the retina. However, the DNA for the Usher 1F protein is too long to fit in the viral carrier. Corey, Indzhykulian and Roska will use three different strategies to overcome this barrier.
Preventing ‘hidden’ hearing loss
HMS neurobiologist Lisa Goodrich is working with neurobiologist and cancer biologist Rosalind Segal and with cancer biologist and pediatric oncologist Loren Walensky, both at HMS and Dana-Farber Cancer Institute, to develop treatments for hidden hearing loss. This slow, insidious form of deafness is estimated to affect more than half of people age 60 and older.
Such hearing loss often occurs as a result of long-term exposure to loud noise that damages the sound-and-motion-sensitive hair cells inside the inner ear. Once damaged, these hair cells do not regenerate, and their gradual demise leads to hearing loss.
A growing body of evidence, however, suggests that hearing loss can also stem from breaks in the connections, or synapses, between the hair cells and the nerves that transmit the auditory signal to the brain. In other words, even when both the hair cells and the signal-conducting nerves are intact and functional, the frayed synapses between them can cause “hidden” hearing loss. When many but not all synapses are lost, for instance, words might be heard but not understood, especially in a noisy room.
A treatment that shields these critical connections from damage—or one that reverses preexisting damage—promises to improve the quality of life for millions of people with noise-induced and age-related hearing loss.
In an effort to protect and restore these connections, Goodrich, professor of neurobiology at HMS, Segal, HMS professor of neurobiology at Dana-Farber, and Walensky, HMS professor of pediatrics at Dana-Farber, are working to develop a therapy that activates a protein known to enhance axon integrity. The therapy builds on previous observations made by the researchers which shows that when this protein is loaded in a viral carrier and delivered to the auditory neurons of the inner ear, it can protect the synapses from noise damage.
The team’s goal is to determine whether delivering this protein could also protect the synapses when delivered after noise exposure. An additional goal of the research is to determine whether the approach could slow down or prevent age-related hearing loss that stems from the loss of synaptic connectivity between hair cells and auditory cells.
Helping the brain make sense of sound
Hearing is essential for navigating the world and for basic survival. This complex process is made possible not only by the detection of sound by hair cells in the inner ear but also by the decoding and interpretation of those signals by specialized brain neurons. This signaling cascade requires the presence of synapses between nerve cells to enable the propagation of the sound signal from one cell to the next.
In early childhood development, these synapses are malleable, meaning they form, disconnect and reform with other neurons until a functional decoding system is established. The phenomenon, known as neural plasticity, allows the maturing brain to develop circuits that can process novel information. As a child grows older, plasticity declines and the neural connections get hardwired. Yet, certain sensory disorders can benefit from restoring some degree of plasticity.
For example, when an adult who has hearing loss receives a cochlear implant—a device that electrically stimulates the nerve from the inner ear to the brain —the brain must rewire some of its circuitry to decipher the new sensory input.
Neuroscientists Bernardo Sabatini, the Alice and Rodman W. Moorhead III Professor of Neurobiology at HMS, and Anne Takesian, HMS assistant professor of otolaryngology at Mass. Eye and Ear, are joining forces to identify ways to boost the plasticity of sound-decoding neurons. The ultimate goal of their work is the development of therapies that enhance neuronal rewiring as a way to boost the efficacy of cochlear implants and to treat age-related hearing loss—a development that could benefit tens of thousands of people each year.
The team’s efforts will build on Takesian’s recent discovery that a small subset of inhibitory neurons regulates the plasticity of the auditory nerve circuitry in development and later life. The work will also capitalize on Sabatini’s expertise in optogenetics—a genetic technique used to render neurons sensitive to activation by light—as well as his expertise in using viruses to illuminate neurons so that the connections among them are revealed.
Toward precision-targeted, non-opioid treatments for acute and chronic pain
Pain disorders take an enormous toll on human health and have been a major driver of the ongoing opioid epidemic. Beyond the physical and psychological suffering that pain inflicts, it cost the U.S. economy more than $600 billion a year in medical care and lost productivity, according to research estimates.
New non-opioid therapies, with no addiction potential and a robust safety profile, are urgently needed to stem the crisis. HMS neurobiologists David Ginty, the Edward R. and Anne G. Lefler Professor of Neurobiology, Bruce Bean, the Robert Winthrop Professor of Neurobiology, and Clifford Woolf, professor of neurobiology at Boston Children’s Hospital, are on a quest to develop such treatments.
The work builds on Ginty’s recent discovery of at least six subtypes of pain-sensing neurons, each of them likely responsible for a different kind of pain, such as joint or skin pain.
Ginty’s lab will focus on identifying unique markers for each pain neuron subtype and on mapping out the signaling trajectory for each, both to identify the body area they sense and the regions of the spinal cord and brain where they send nerve signals.
Bean’s team aims to unravel the molecular profile of each of these neuronal subtypes to identify the proteins on their surface that are good drug targets. Ginty, together with Woolf’s lab at Boston Children’s, will work to optically activate or silence each neuronal subtype to understand what kind of pain it produces.
Together, the team’s findings will provide a detailed structural and functional profile of each one of these distinct types of pain neurons, paving the way to precision therapies that target each specific type of pain.
The Bertarelli Program in Translational Neuroscience and Neuroengineering was launched with a gift from the Bertarelli Foundation in 2010 to address some of the most important questions in medical neuroscience that, once solved, would have life-changing outcomes for patients across the globe. Its focus is not just on stimulating new cross-disciplinary research but also on establishing cross-border and cross-institutional working communities for knowledge sharing. The aim of these collaborations is to bridge the gap between basic and translational neuroscience by supporting basic and clinical research oriented toward translational opportunities; by creating stronger ties among scientists, engineers and clinicians; and by training the next generation of leaders in the field.
Harvard Medical School has more than 11,000 faculty working in 10 academic departments located at the School’s Boston campus or in hospital-based clinical departments at 15 Harvard-affiliated teaching hospitals and research institutes: Beth Israel Deaconess Medical Center, Boston Children’s Hospital, Brigham and Women’s Hospital, Cambridge Health Alliance, Dana-Farber Cancer Institute, Harvard Pilgrim Health Care Institute, Hebrew SeniorLife, Joslin Diabetes Center, Judge Baker Children’s Center, Massachusetts Eye and Ear/Schepens Eye Research Institute, Massachusetts General Hospital, McLean Hospital, Mount Auburn Hospital, Spaulding Rehabilitation Network and VA Boston Healthcare System.